
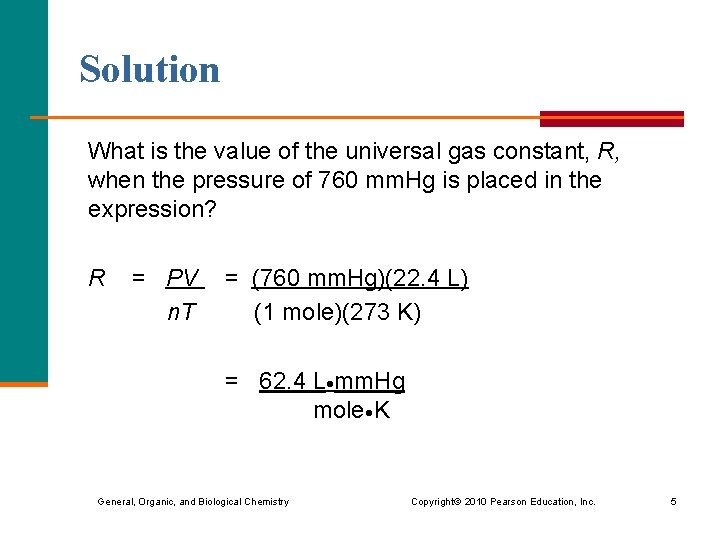
It is important for determining the relationship between the amount of gas (N) and the volume of the gas (V). 1 mole of any gas at NTP occupies a volume of 22.4L.
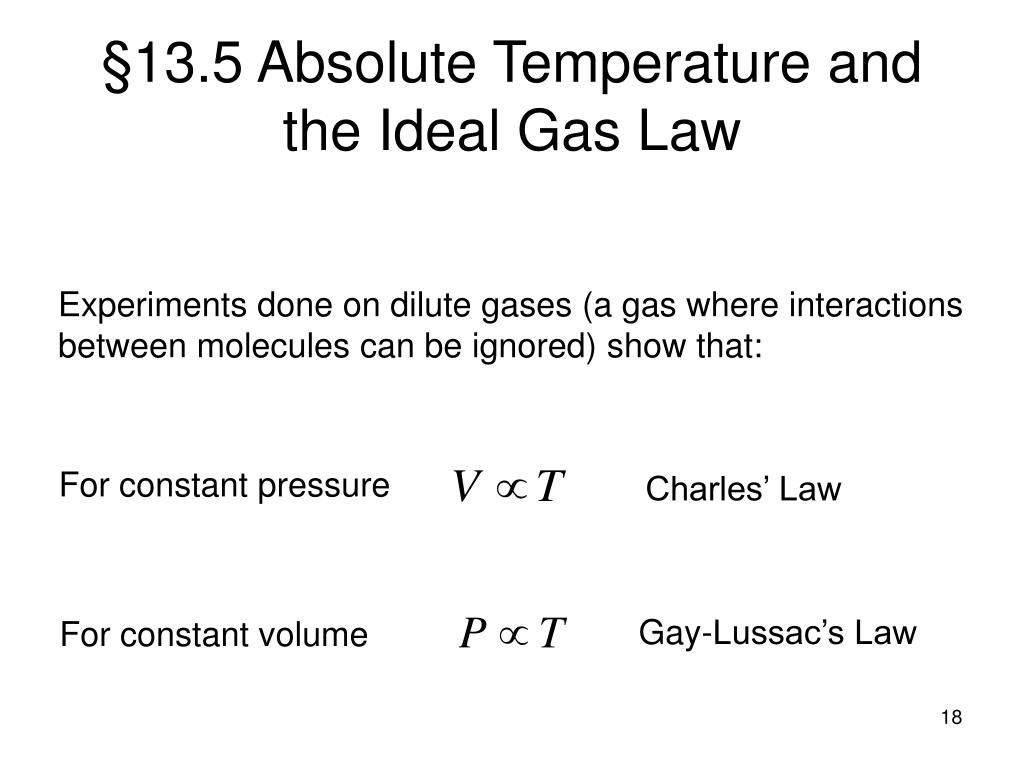
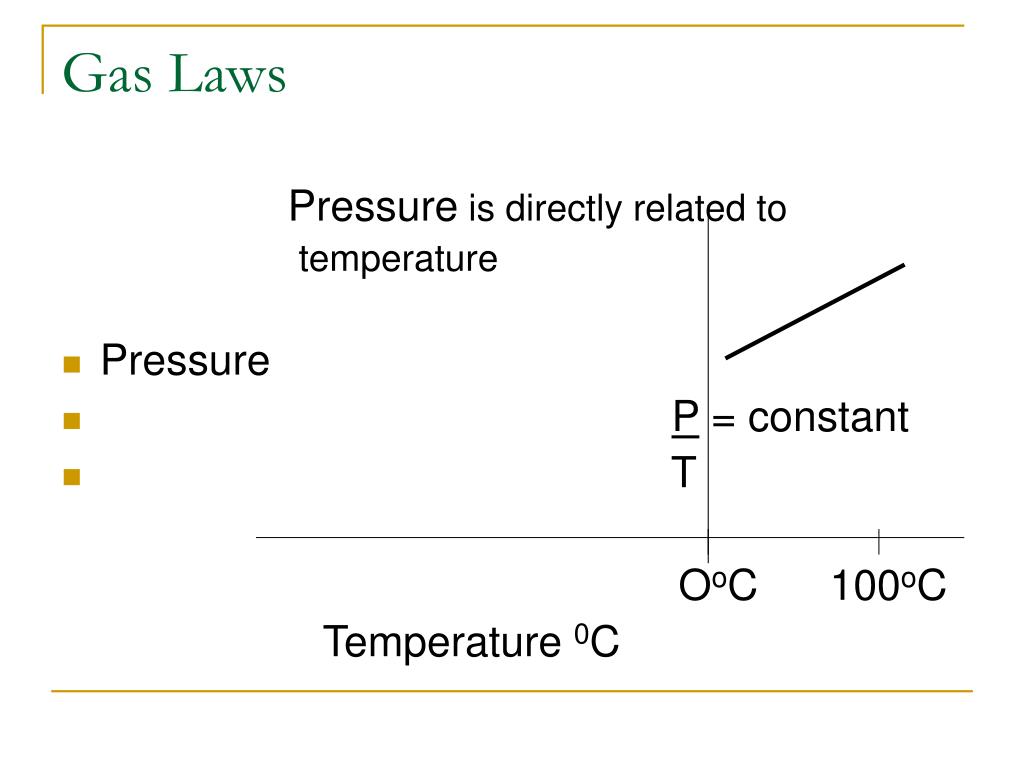
As the temperature increases, the pressure inside the pressure cooker also increases, which makes the food cook faster.Īvogadro’s law states that for constant temperature, pressure, and volume, all the gases contain an equal number of molecules. Under the similar condition, the initial and final pressure and temperature for a given volume of gas can be calculated Įxample: The working of a pressure cooker follows the Gay Lussac’s law. Now as c^2 ∝ T, thus at a constant volume, P ∝ T for a given mass of gas.Īs the temperature increases, the pressure also increases. Gay Lussac’s Law states that at a constant volume, the pressure of a given mass of a gas is directly proportional to its absolute temperature i.e., at constant volume, P ∝ T or P/T= constant. Gay Lussac’s Law: The Pressure-Temperature Law This proves that under constant pressure conditions, if there is a fall in temperature, the volume also decreases. You will notice that when the ball is left under colder conditions, it starts losing the air inside it or its volume starts decreasing. Moreover, the initial and final temperature, as well as the volume of a gas, can be easily determined Įxample: Leaving a basketball out during the cold months deflates it. Proof: Now as c 2 ∝ T, thus at a constant pressure for a given mass of a gas, V ∝ T.Īs the temperature of a gas increases, the volume of the gas also increases. As the tire is inflated with more and more air at the same temperature, all the molecules of gas are forced to pack together, reduce their volume, and increase the pressure on the walls of the tire.Ĭharles Law or Law of Volume states that at constant pressure, the volume of a given mass of a gas is directly proportional to its absolute temperature i.e., at constant pressure, V ∝ T or V/T= constant. The following equation can be derived from the Boyle’s Law:Įxample: When compressed air is filled in a tire, the pressure measurements are taken into consideration. When the pressure increases, the volume of a gas decreases and vice-versa. Now, at a constant temperature, c, N, and m 1 are constants hence, Where c is the root mean square velocity of the molecules, m1 is the mass of a molecule, V is the volume, and N is the number of molecules. Proof: From the Kinetic Theory of gases, we know: The state variables of the gas are:īoyle’s Law states that at a constant temperature, the volume of a given mass of a gas is inversely proportional to the pressure i.e., at constant temperature V ∝ 1/P or PV= constant.
Different scientists did numerous experiments and hence, put forth different gas laws which relate to different state variables of a gas. The ‘Kinetic Theory of Gases’ derives the ‘Equation of State’ for an ideal gas. Gases have widely spaced individual particles. Nonetheless, all the gases behave similarly. These relationships would, in turn, be, approximately, valid for all the gases. The gas laws were developed in the late 1800s when the scientists understood the relationship between the pressure, volume, and temperature for a sample of gas.


 0 kommentar(er)
0 kommentar(er)
